RiaSTAP is a lyophilized fibrinogen concentrate (coagulation factor
I), made from human plasma, and administered intravenously. It has been used for
years in several European countries under the name Haemocomplettan®
P.
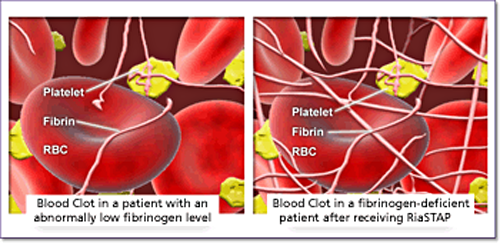
In patients with CFD, administered RiaSTAP replaces absent or low
fibrinogen. As such, RiaSTAP serves as a physiological substrate
of thrombin (factor IIa), which converts soluble fibrinogen to insoluble fibrin.
Under the influence of factor XIIIa, fibrin strands are cross-linked to provide
strength and stability to the blood clot—fulfilling an essential need for
clot formation in patients with fibrinogen deficiency.
Important Safety Information
RiaSTAP®, Fibrinogen Concentrate (Human), is contraindicated in patients with known anaphylactic or severe systemic reactions to human plasma-derived products.
Monitor patients for early signs of anaphylaxis or hypersensitivity reactions and if necessary, discontinue administration and institute appropriate treatment. Thrombotic events have been reported in patients receiving RiaSTAP; weigh the benefits of administration versus the risks of thrombosis.
RiaSTAP is made from human blood. The risk of transmission of infectious agents, including viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent and its variant (vCJD), cannot be completely eliminated.
The most serious adverse reactions observed are thrombotic episodes (pulmonary embolism, myocardial infarction, deep vein thrombosis) and anaphylactic reactions. The most common adverse reactions observed in clinical studies (frequency >1%) were fever and headache.
Indications
RIASTAP is indicated for the treatment of acute bleeding episodes in pediatric and adult patients with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia.
Please see full prescribing information for RiaSTAP.
To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.