RiaSTAP dosing, duration of dosing, and frequency of administration should be individualized based
on the extent of bleeding, laboratory values, and the clinical condition of the patient.
When the baseline fibrinogen level is known, the dose should be individually calculated for each
patient. When the baseline fibrinogen level is not known, the recommended dose is 70 mg/kg of body
weight administered intravenously.
Monitoring of patient’s fibrinogen level is recommended during treatment with RiaSTAP. A target
fibrinogen level of 100 mg/dL should be maintained until hemostasis is obtained.
RiaSTAP must be reconstituted prior to use. The injection rate should not exceed 5 mL per minute.
| Dosing Guidelines |
| If the fibrinogen level is: |
The recommended dose of RiaSTAP is: |
| Known |
Target level (mg/dL) - measured level (mg/dL)
1.7 (mg/dL per mg/kg body weight)
Example when fibrinogen is known
- Target level = 100 mg/dL
- Patient's measured level = 20 mg/dL
- Patient's weight = 70 kg
Dose of RiaSTAP
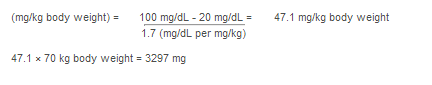
|
| Unknown |
70 mg/kg of body weight |
- RiaSTAP half-life (hours) = 78.7 ± 18.13 (Mean +/- SD)
Learn about the
Convenience of RiaSTAP
See an interactive overview of the
Coagulation Cascade.
Important Safety Information
RiaSTAP®, Fibrinogen Concentrate (Human), is contraindicated in patients with known anaphylactic or severe systemic reactions to human plasma-derived products.
Monitor patients for early signs of anaphylaxis or hypersensitivity reactions and if necessary, discontinue administration and institute appropriate treatment. Thrombotic events have been reported in patients receiving RiaSTAP; weigh the benefits of administration versus the risks of thrombosis.
RiaSTAP is made from human blood. The risk of transmission of infectious agents, including viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent and its variant (vCJD), cannot be completely eliminated.
The most serious adverse reactions observed are thrombotic episodes (pulmonary embolism, myocardial infarction, deep vein thrombosis) and anaphylactic reactions. The most common adverse reactions observed in clinical studies (frequency >1%) were fever and headache.
Indications
RIASTAP is indicated for the treatment of acute bleeding episodes in pediatric and adult patients with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia.
Please see full prescribing information for RiaSTAP.
To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.